It is an automated assay that utilizes isothermal nucleic acid amplification technology. The Abbott ID NOW COVID-19 ID NOW COVID-19 assay is a molecular test used to detect the part of the SARS-CoV-2 virus called viral RNA nucleic acid which is the viruss genetic material.
 Abbott Id Now Covid 19 Instructions Modified Due To R
Abbott Id Now Covid 19 Instructions Modified Due To R
In search for a platform with a shorter turnaround time we sought to evaluate the recently released Abbott ID Now COVID-19 assay.

Id now test. Food and Drug Administration FDA f. The ID NOW COVID-19 rapid test delivers high-quality molecular positive results in as little as 5 minutes. Food and Drug Administration FDA for the ID NOW COVID-19 test in March 2020.
It has been available within MemorialCare for about two weeks and there remains some confusion about its proper use in testing. Our unique ID NOW isothermal nucleic acid amplification technology provides molecular results in just minutes allowing you to make effective clinical decisions sooner. ID NOW is one of the most accurate technologies available for testing results in 20 minutes and identifying novel Coronavirus.
The Abbott ID Now test utilizes a nucleic acid molecular detection method similar to. ID NOW Test is the fasted diagnostic test for the detection of COVID-19 that uses the ID NOW platform of the Abbott Company. The ID NOW COVID-19 test is a rapid molecular point-of-care test that detects COVID-19 in 13 minutes or less.
Abbott received emergency use authorization EUA from the US. Health Canada regulators today approved the ID NOW rapid COVID-19 testing device for use in this country a move that could result in millions more tests. Molecular tests are different from antigen tests.
However the run times for each assay are 35 h and 45 min respectively. The two platforms demonstrate comparable performances. ID NOW COVID-19 is a rapid 13 minutes or less instrument-based isothermal test for the qualitative detection and diagnosis of -CoV-SARS2 from nasal nasopharyngeal and throat swabs.
The ID NOW COVID-19 should be ordered for the detection of COVID-19 in individuals who are. On 15 May the FDA released an alert regarding the Abbott ID NOW test for Covid-19 and its potential to provide inaccurate negative results. ID NOW is an FDA approved CLIA-waived instrument which means.
The diagnostic test which runs for 5 minutes for a positive test and for 13-15 minutes for a negative test. The Abbott ID Now Rapid Molecular Test for COVID-19 is the first in-house lab testing available to MemorialCare. ID NOW is a rapid instrument-based isothermal system for the qualitative detection of infectious diseases.
The test is an automated assay that delivers positive results in just five minutes and takes only 13 minutes to show the negative results using the ID NOW molecular platform. Find out more about this innovative technology and its impact here. Tes lebar pita koneksi internet Anda ke lokasi-lokasi di seluruh dunia dengan tes kecepatan jalur lebar interaktif ini.
ID Now COVID-19 Abbott Diagnostics Scarborough Inc Scarborough ME is a rapid test that qualitatively detects SARS-CoV-2 viral nucleic acids from nasal nasopharyngeal and throat swabs. The ID NOW molecular platform of the test is an instrument-based isothermal system for rapid qualitative identification of infectious diseases. What is the Abbott ID NOW COVID-19 assay.
ID Now COVID-19 Abbott Diagnostics Scarborough Inc Scarborough ME is a rapid test that qualitatively detects SARS-CoV-2 viral nucleic acids from nasal nasopharyngeal and throat swabs. This sensitivity among other concerns should be taken into consideration when using this test for patients with a low suspicion for COVID-19 disease. It is used on our ID NOW platform.
Go inside molecular testing and see how ID NOW worksAbbott has received emergency use authorization EUA from the US. Abbott Laboratories is now advising customers that its ID NOW device should be used with dry nasal swabs instead of viral transport media. The portable rapid molecular ID NOW COVID-19 test has emerged as a critical part of this arsenal allowing fast diagnosis with results in 13 minutes or less in a variety of locations such as physicians offices urgent care clinics and other point-of-care locations.
COVID-19 PCR Testing - Standard Swab Test. The ID NOW COVID-19 can be used to test direct nasal nasopharyngeal or throat swabs. ID NOW is a leading molecular point-of-care platform in the United States trusted by hospitals physician offices and urgent care clinics nationwide.
ID NOW performs well for strong and moderately positive samples but has reduced sensitivity for weakly positive samples.
 Abbott Id Now Covid 19 Detection Test System Us
Abbott Id Now Covid 19 Detection Test System Us
Https Www Fda Gov Media 136525 Download
 Indonesia Go Id Cartridge Nya Isi Ulang Diagnosisnya Lima Menit
Indonesia Go Id Cartridge Nya Isi Ulang Diagnosisnya Lima Menit
 Abbott Launches Molecular Point Of Care Test To Detect Novel Coronavirus In As Little As Five Minutes Mar 27 2020
Abbott Launches Molecular Point Of Care Test To Detect Novel Coronavirus In As Little As Five Minutes Mar 27 2020
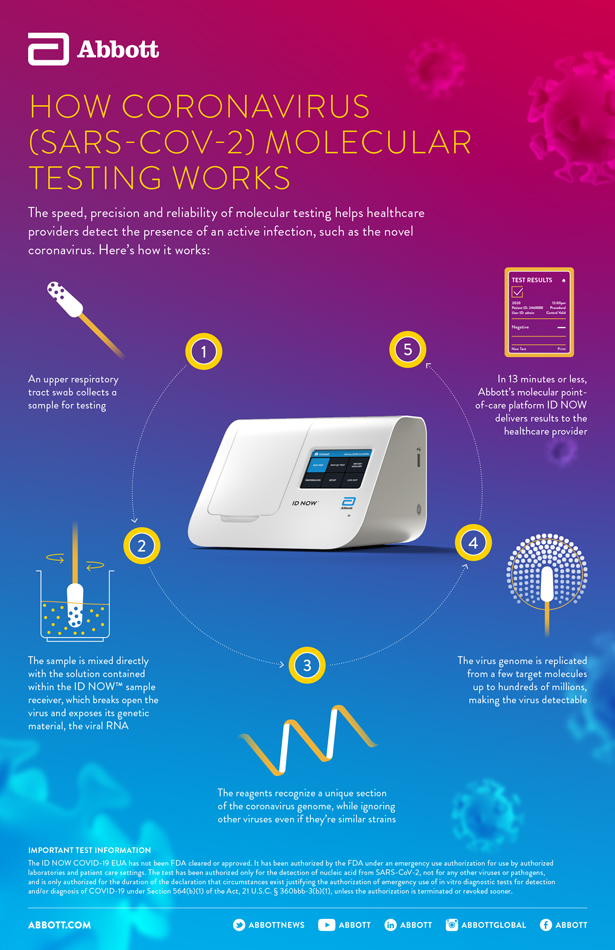 How Id Now Tackles Covid 19 Abbott Newsroom
How Id Now Tackles Covid 19 Abbott Newsroom
 Id Now Abbott Point Of Care Testing
Id Now Abbott Point Of Care Testing
 Id Now Abbott Point Of Care Testing
Id Now Abbott Point Of Care Testing
 As Problems Grow With Abbott S Fast Covid Test Fda Standards Are Under Fire Kaiser Health News
As Problems Grow With Abbott S Fast Covid Test Fda Standards Are Under Fire Kaiser Health News
 Steps To Use Id Now Effectively Abbott Newsroom
Steps To Use Id Now Effectively Abbott Newsroom
 Abbott Id Now Covid 19 Results More Accurate With Earlier Testing Massdevice
Abbott Id Now Covid 19 Results More Accurate With Earlier Testing Massdevice
 N J Gets 15 Abbott Id Now Machines The Ones That Can Do Covid 19 Tests In As Little As 5 Minutes Roi Nj
N J Gets 15 Abbott Id Now Machines The Ones That Can Do Covid 19 Tests In As Little As 5 Minutes Roi Nj
 First In Indonesia The Detection Of Covid 19 By Isothermal Molecular Test Bali Plus Magazine
First In Indonesia The Detection Of Covid 19 By Isothermal Molecular Test Bali Plus Magazine
 Rapid Tests For Covid 19 Are Missing Early Infections Expert Warns The Japan Times
Rapid Tests For Covid 19 Are Missing Early Infections Expert Warns The Japan Times
 Id Now The Forefront Of Covid 19 Testing Abbott U S
Id Now The Forefront Of Covid 19 Testing Abbott U S
No comments:
Post a Comment
Note: Only a member of this blog may post a comment.