When Regenerons co-Founder President and Chief Scientific Officer George D. Regenerons VelocImmune technology utilizes a proprietary genetically engineered mouse platform endowed with a genetically humanized immune system to produce optimized fully human antibodies.
Fhn Treats Covid 19 Patients With Monoclonal Antibodies
Food and Drug Administration FDA has granted an Emergency Use Authorization EUA for REGEN-COV casirivimab and imdevimab to be administered together for the treatment of mild-to-moderate COVID-19 in adults as well as in pediatric patients weighing at least 40 kg who are at least 12 years of age with positive results of direct SARS-CoV-2 viral testing and who are at high risk for.
Regeneron monoclonal antibody. Regenerons antibody cocktail is made up of two different monoclonal antibodies casirivimab and imdevimab. The REGEN-COV antibodies were made in a lab by the pharmaceutical company Regeneron. Approval for its COVID-19 monoclonal antibody cocktail as a preventative treatment after it helped cut the risk of.
It is an artificial antibody cocktail designed to produce resistance to the SARS-CoV-2coronavirusresponsible for the COVID-19 pandemic. Alt in 1985. ZURICH Reuters - Regeneron is pursuing US.
R egeneron said Tuesday that its monoclonal antibody cocktail prevented Covid-19 in a clinical trial. Original Article from The New England Journal of Medicine REGN-COV2 a Neutralizing Antibody Cocktail in Outpatients with Covid-19. New Phase 3 trial data finds a single shot of Regenerons Covid-19 antibody cocktail was able to prevent symptomatic Covid-19 among people exposed to the virus the company said Monday.
Last week pharma company Eli Lilly revealed its monoclonal antibody treatment for COVID-19 is 80 percent effective at preventing viral infection. November 23 2020 -- The FDA issued an emergency use authorization to Regeneron Pharmaceuticals for its monoclonal antibodies -- casirivimab and imdevimab --. The FDA EUA requires both to be administered together via intravenous IV infusion.
The combination of two antibodies is intended to prevent mutational escape. CDC warns new Covid-19 variants could accelerate spread in US 0215 CNN Tests on two different variants of the coronavirus show that one of the two monoclonal antibodies. About Regeneron Antibody Cocktail Casirivimab and imdevimab injection is a cocktail of two monoclonal antibodies also known as REGN10933 and REGN10987 respectively and was designed specifically to block infectivity of SARS-CoV-2 the virus that causes COVID-19.
A monoclonal antibody treatment developed by the drug maker Regeneron sharply cut the risk of hospitalization and death when given to high-risk Covid-19 patients in a large clinical trial. It consists of two monoclonal antibodies casirivimab REGN10933 and imdevimab REGN10987 that must be mixed together. While most of our approved medicines are fully human monoclonal antibodies we are exploring newer types of antibody medicines including bispecific antibodies and antibody drug conjugates ADCs.
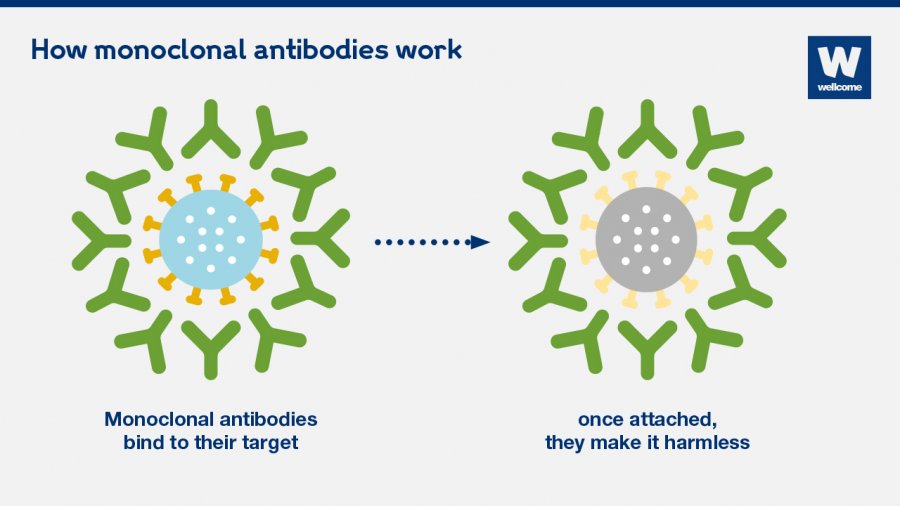
Regenerons therapy is part of a class of treatments known as monoclonal antibodies which are made to act as immune cells and fight infections. His antibody cocktail did not use human fetal cells. A monoclonal antibody is a laboratory-made clone of a specific parent immune cell intended to fight infection in the body.
Learn more about the Regeneron. Getty President Donald Trump returns to the White House from Walter Reed National Military Medical Center on October 5 2020. Yancopoulos was a graduate student with his mentor Frederick W.
The news issued via a press release mirrored similar news from Eli Lilly last week that its. The REGEN-COV antibody cocktail cannot give you SARS-CoV-2 nor will they make you sick with COVID-19. Regeneron is leading the way developing new technologies to help discover better and novel antibody medicines.
Not to be outdone Lillys major monoclonal.