It also reduced the incidence of postherpetic neuralgia the most common. Skip to content Sign up for our monthly Lifestyle newsletter for entertainment news healthy living tips and more.
 What Makes The Pfizer And Moderna Vaccines So Promising
What Makes The Pfizer And Moderna Vaccines So Promising
The vaccine combines an antigen glycoprotein E and an adjuvant system AS01B intended to generate a strong and long-lasting immune response that can help overcome the decline in immunity that.

When was shingrix released. Shingrix FDA Approval History. Shingrix is the first new shingles vaccine in more than a decade and only the second to ever be approved Zostavax was the first by the Food and Drug Administration. Talk to your healthcare provider to determine the best time to get Shingrix.
Shingrix GSK is an adjuvanted subunit vaccine con-sistingofasinglerecombinant VZV antigenglycoprotein gE and the AS01 B adjuvant system 12. The Product Information is intended to assist healthcare professionals make decisions about treatment options and provide advice. Shingrix was approved in the US vii and Canada viii in October 2017 and has been recommended by the US Centers for Disease Control and Preventions Advisory Committee on Immunization Practices as the preferred vaccine for the prevention of herpes zoster and related complications for immunocompetent adults aged 50 years and older.
The FDA approval marks the second regulatory green light for the vaccine in a weeks time. GlaxoSmithKline says Shingrix will be available shortly Zostavax was licensed and recommended by the committee in. Zoster vaccine recombinant adjuvanted Dosage form.
October 20 2017 Approval Letter - SHINGRIX. If you had Zostavax in the past you should still get Shingrix. It was approved in.
MississaugaON - SHINGRIX has been approved in Canada for the prevention of shingles herpes zoster in people aged 50 years or older. Shingrix Herpes Zoster vaccine recombinant AS01Bxiiii adjuvanted is a non-live recombinant subunit vaccine to help prevent shingles herpes zoster in adults 50 years of age and older. Shingrix a new shingles vaccine approved by the FDA in 2017 is now widely available across the country in pharmacies and with physicians across.
On 13 October 2017 Shingrix was approved in Canada for the prevention of shingles herpes zoster in people aged 50 years or older. The dates of manufacture of the VZV gE antigen and the AS01 B. Last Friday Shingrix was approved for sale in Canada.
0 to 50 years SAEs were reported for 128 of subjects who received SHINGRIX and for 133 of subjects who received placebo. A shingles vaccine called Zostavax is no longer available for use in the United States as of November 18 2020. The safety of Shingrix was evaluated by pooling data from 2 placebo-controlled clinical studies Studies 1 and 2 involving 29305 subjects aged 50 years and older who received at least 1 dose of Shingrix n 14645 or saline placebo n 14660 administered according to a 0-.
May 17 2019 Approval Letter - SHINGRIX. March 24 2021 Approval Letter - SHINGRIX. Following this approval from FDA and pending a recommendation from ACIP Shingrix will be available shortly.
September 18 2018 Approval Letter - SHINGRIX. Adjuvant component of SHINGRIX is 36 months from the date of manufacture when stored at 2-8º C. Regulatory filings in the European Union Australia and Japan are underway.
During the entire follow-up period median 44 years range. Additionally the committee expressed its preference for Shingrix over Zostavax. Release This stimulates NK cells and CD8 T cells to interferon- gIFN- which in turn stimulates activation and recruitment of blood monocyte-derived and.
Yes First approved October 20 2017 Brand name. On 13 October 2017 Shingrix was approved in Canada for the prevention of shingles herpes zoster in people aged 50 years or older. Recombinant Varicella Zoster Virus glycoprotein E antigen AS01 B adjuvanted vaccine Product Information.
In October the Advisory. SHINGRIX 23 and placebo 22 within 30 days after the last dose of vaccine or placebo. Regulatory filings are also in the works for the.
If you have questions about Shingrix talk with your healthcare provider. 13 2019 -- Unlike some vaccines theres been so much demand for the new shingles vaccine Shingrix that its not always easy to find. Regulatory filings in the European Union Australia and Japan.
In a pooled analysis Shingrix was more than 90 effective at preventing herpes zoster or shingles the release said. I SHINGRIX is a non-live recombinant subunit adjuvanted vaccine given intramuscularly in two doses and is expected to be available through physicians offices and pharmacies in early 2018.
 A New Shingles Vaccine Is Now Available Nationwide
A New Shingles Vaccine Is Now Available Nationwide
 New Shingrix Vaccine For Shingles Olympic Internal Medicine Inc P S
New Shingrix Vaccine For Shingles Olympic Internal Medicine Inc P S
 Why The Latest Shingles Vaccine Is More Than 90 Effective Scimex
Why The Latest Shingles Vaccine Is More Than 90 Effective Scimex
 Shingrix Everything You Need To Know About The New Shingles Vaccine
Shingrix Everything You Need To Know About The New Shingles Vaccine
 Shingles Vaccine What Are The Side Effects
Shingles Vaccine What Are The Side Effects
 Should I Get The New Shingles Vaccine Science Based Medicine
Should I Get The New Shingles Vaccine Science Based Medicine
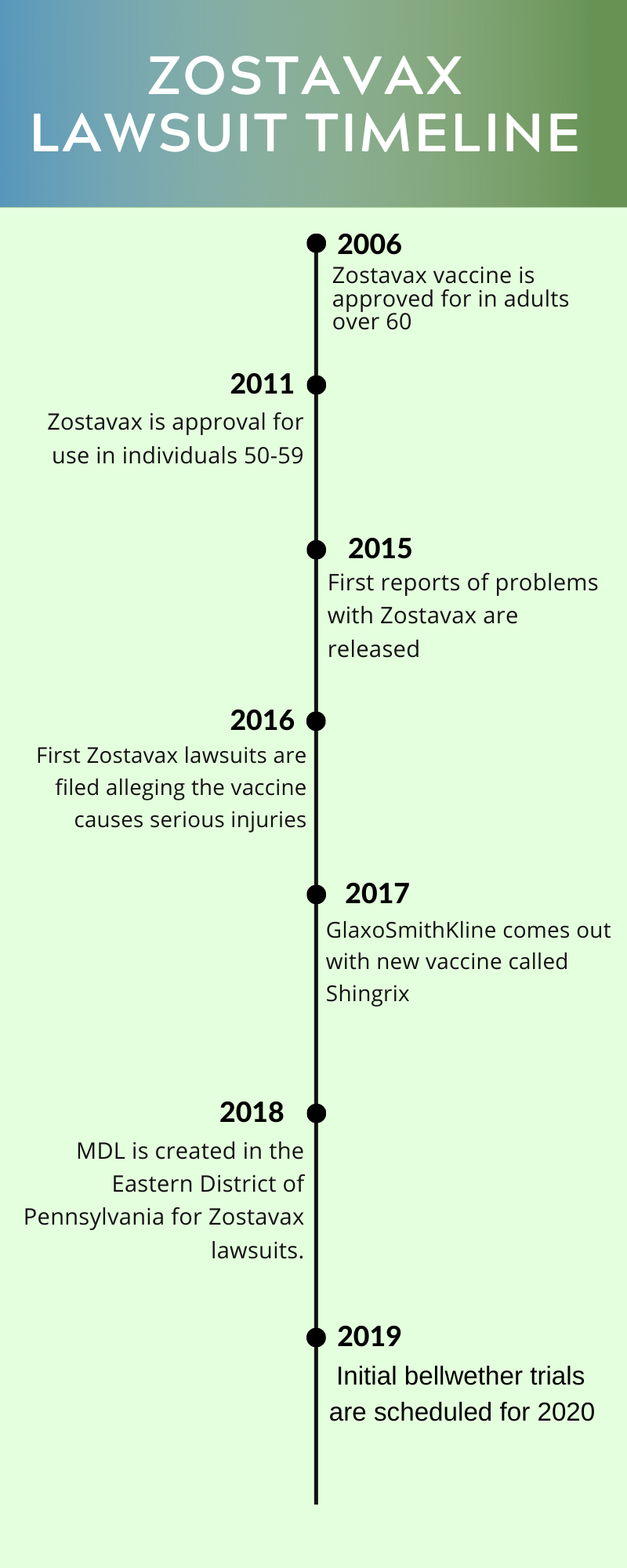 Shingles Lawsuit October 2020 Update Federal Court Trials On Tap
Shingles Lawsuit October 2020 Update Federal Court Trials On Tap
 Zoster Vaccine Recombinant Adjuvanted Shingrix
Zoster Vaccine Recombinant Adjuvanted Shingrix
 New Shingles Vaccine Bremo Pharmacy
New Shingles Vaccine Bremo Pharmacy
 Shingrix Sunray Drugs Specialty Pharmacy
Shingrix Sunray Drugs Specialty Pharmacy
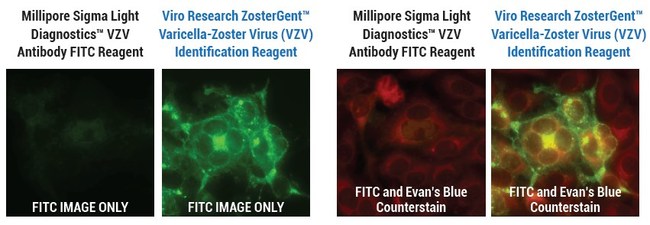 Inventor Of Shingrix Develops New Chickenpox And Shingles Virus Detection Test
Inventor Of Shingrix Develops New Chickenpox And Shingles Virus Detection Test
 Shingles Vaccine Pros And Cons Vitruviamd Dr Laura Miles
Shingles Vaccine Pros And Cons Vitruviamd Dr Laura Miles
 Shingle Vaccination Rate Soars But Cost Leaves Many Behind Health Unionleader Com
Shingle Vaccination Rate Soars But Cost Leaves Many Behind Health Unionleader Com
No comments:
Post a Comment
Note: Only a member of this blog may post a comment.